Prostaglandin HPLC Mixture
Prostaglandin HPLC Mixture
This mixture contains primary prostaglandins produced from arachidonic acid and dihomo-?-linolenic acid. Contents: Prostaglandin E1, Prostaglandin E2, Prostaglandin F1?, 6-keto...
From €231.00 €196.35
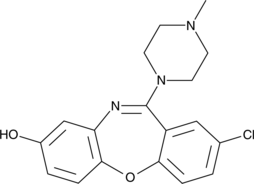
8-hydroxy Loxapine (8-OH loxapine) is a metabolite formed when loxapine (Item No. 20760), an atypical antipsychotic, is metabolized by the cytochrome P450 isoform CYP1A2.{24252,36038} Loxapine displays high affinity for histamine, serotonin (5-HT), dopamine, and ?1-adrenergic receptors (Ki values = 7, 7.7, 9.5, 12, and 31 nM for H1, 5-HT2A, 5-HT2C, D2, and ?1A-adrenergic receptors, respectively).{24252,24253} It reduces agitation associated with schizophrenia or bipolar disorder.{32724} 8-OH Loxapine is considered inactive as it has relatively low affinity to dopamine and 5-HT receptors compared to the parent compound, however, 8-OH loxapine inhibits [14C]5-HT uptake in vitro (IC50 = 2 ?M in human platelets).{36039}
Territorial Availability: Available through Bertin Technologies only in France
| Size | 1 mg, 500 µg |
|---|---|
| Shipping | dry ice |
| CAS number | 61443-77-4 |
| Molecular formula | C18H18ClN3O2 |
| Smiles | ClC1=CC2=C(C=C1)OC3=C(C=C(O)C=C3)N=C2N4CCN(C)CC4 |
| Molecular weight | 343,8 |
| Custom code | 2903.99 |
| Formulation | A solid |
| Purity | ≥98% |
| UNSPSC code | 12352100 |
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.
This mixture contains primary prostaglandins produced from arachidonic acid and dihomo-?-linolenic acid. Contents: Prostaglandin E1, Prostaglandin E2, Prostaglandin F1?, 6-keto...
Protein phosphorylation is an important post-translational modification that serves many key functions to regulate a protein’s activity, localization, and protein-protein...
CD34 is a transmembrane phosphoglycoprotein and sialomucin protein that is commonly used as a marker for hematopoietic progenitor cells.{59706,59704} It...
This mixture contains the characteristic metabolites of both PGI2 and TXA2. Contents: Thromboxane B2, 11-dehydro Thromboxane B2, 6-keto Prostaglandin F1?,...
The cyclopentenone prostaglandin HPLC mixture contains all of the major UV-absorbing cyclopentenone prostaglandins and their precursors supplied in methyl acetate....
Proprotein convertase subtilisin kexin 9 (PCSK9) is a member of the subtilisin serine protease family with an important role in...
(+)-D-threo-PDMP is a ceramide analog and is one of the four possible stereoisomers of PDMP (Item No. 62595).{11392} (+)-D-threo-PDMP is...
Cytosolic PGE synthase (cPGES) is a glutathione-dependent enzyme with a predicted size of 18.6 kDa (23 kDa on SDS-PAGE). The...
Endocannabinoids, such as arachidonoyl ethanolamide (AEA) and 2-arachidonoyl glycerol (2-AG), function as short-range modulators of cell and synaptic activity. Monoacylglycerol...
This rabbit anti-mouse IgA FITC is used as the ‘secondary antibody’ for immunostaining experiments where the primary antibody is mouse...
This mixture contains the primary metabolites of prostaglandins (PGs) D2, E2, and F2?. Contents: 13,14-dihydro-15-keto PGD2, 13,14-dihydro-15-keto PGE2, 11?-PGF2?, 13,14-dihydro-15-keto...
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a basic leucine zipper transcription factor encoded by NFE2L2 in humans that...
GST-tag Polyclonal Antibody (FITC) is a probe for the immunochemical detection of GST tags on recombinant proteins. Recombinant proteins are...
This mixture contains the primary COX products produced by most mammalian tissues. Contents: Prostaglandin D2, Prostaglandin E2, 6-keto Prostaglandin F1?,...
GPR17 is a G protein-coupled receptor that has been identified as a dualistic receptor recognizing signals from two unrelated chemical...
The Cayman COX Inhibitor Pack contains a combination of frequently used cyclooxygenase (COX) inhibitors. Each kit contains aspirin, the archetype...
Adenosine Receptor A2A is a multi-pass membrane protein that is normally localized to the plasma membrane.{15465} This receptor is part...
24(S),25-epoxy Cholesterol is an oxysterol and the most abundant oxysterol in mouse ventral midbrain.{43943} It activates liver X receptors (LXRs)...