FABP3 Blocking Peptide
FABP3 Blocking Peptide
To be used in conjunction with Cayman’s FABP3 polyclonal antibody (Catalog No. 10233) to block protein-antibody complex formation during immunochemical...
From €243.00 €206.55
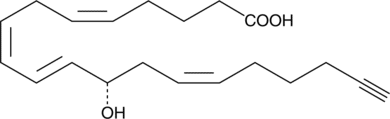
12(S)-HETE (Item No. 34570) is the predominant lipoxygenase product of mammalian platelets.{498} It enhances tumor cell adhesion to endothelial cells, fibronectin, and the subendothelial matrix at 0.1 µM.{591,880} 12(S)-HETE-19,20-alkyne is a form of 12(S)-HETE with an ?-terminal alkyne. The terminal alkyne group can be used in click chemistry linking reactions, to tag 12(S)-HETE with fluorescent or biotinylated labels for analysis of its metabolism and biological activity.{17991,17992}
Territorial Availability: Available through Bertin Technologies only in France
| Size | 100 µg, 25 µg, 50 µg |
|---|---|
| Shipping | dry ice |
| Molecular formula | C20H28O3 |
| Smiles | C#CCCC/C=CC[C@H](O)/C=C/C=CC/C=CCCCC(O)=O, C#CCCC/C=CC[C@H](O)/C=C/C=CC/C=CCCCC(O)=O, C#CCCC/C=CC[C@H](O)/C=C/C=CC/C=CCCCC(O)=O |
| Molecular weight | 316,4 |
| Custom code | 2916.19 |
| Formulation | A solution in ethanol |
| Purity | ≥97% |
| UNSPSC code | 12352100 |
Cayman Chemical’s mission is to help make research possible by supplying scientists worldwide with the basic research tools necessary for advancing human and animal health. Our utmost commitment to healthcare researchers is to offer the highest quality products with an affordable pricing policy.
Our scientists are experts in the synthesis, purification, and characterization of biochemicals ranging from small drug-like heterocycles to complex biolipids, fatty acids, and many others. We are also highly skilled in all aspects of assay and antibody development, protein expression, crystallization, and structure determination.
Over the past thirty years, Cayman developed a deep knowledge base in lipid biochemistry, including research involving the arachidonic acid cascade, inositol phosphates, and cannabinoids. This knowledge enabled the production of reagents of exceptional quality for cancer, oxidative injury, epigenetics, neuroscience, inflammation, metabolism, and many additional lines of research.
Our organic and analytical chemists specialize in the rapid development of manufacturing processes and analytical methods to carry out clinical and commercial GMP-API production. Pre-clinical drug discovery efforts are currently underway in the areas of bone restoration and repair, muscular dystrophy, oncology, and inflammation. A separate group of Ph.D.-level scientists are dedicated to offering Hit-to-Lead Discovery and Profiling Services for epigenetic targets. Our knowledgeable chemists can be contracted to perform complete sample analysis for analytes measured by the majority of our assays. We also offer a wide range of analytical services using LC-MS/MS, HPLC, GC, and many other techniques.
Accreditations
ISO/IEC 17025:2005
ISO Guide 34:2009
Cayman is a leader in the field of emerging drugs of abuse, providing high-purity Schedule I-V Controlled Substances to federally-licensed laboratories and qualified academic research institutions for forensic analyses. We are certified by ACLASS Accreditation Services with dual accreditation to ISO/IEC 17025:2005 and ISO Guide 34:2009.
To be used in conjunction with Cayman’s FABP3 polyclonal antibody (Catalog No. 10233) to block protein-antibody complex formation during immunochemical...
Proprotein convertase subtilisin kexin 9 (PCSK9) is a member of the subtilisin serine protease family with an important role in...
Amyloid-? (1-8, A2V) is a truncated form of amyloid-? (A?) that contains a valine to alanine substitution at position 2...
Histone H2A is a core histone that forms a dimer with histone H2B.{46631} Two histone H2A/H2B dimers form an octameric...
Sirtuin 5 (SIRT5) is an enzyme that catalyzes the NAD-dependent removal of malonyl, succinyl, and glutaryl groups from target proteins.{55107,55108}...
This mixture contains the primary COX products produced by most mammalian tissues. Contents: Prostaglandin D2, Prostaglandin E2, 6-keto Prostaglandin F1?,...
Inositol hexakisphosphate kinase 2 (IP6K2) is a cytoplasmic kinase that catalyzes the conversion of IP6 to diphosphoinositol pentakisphosphate in the...
The Cayman COX Inhibitor Pack contains a combination of frequently used cyclooxygenase (COX) inhibitors. Each kit contains aspirin, the archetype...
Programmed cell death protein 4 (PDCD4) levels are elevated during apoptosis and absent in many cancer samples.{16340,16167} Loss of PDCD4...
This mixture contains primary prostaglandins produced from arachidonic acid and dihomo-?-linolenic acid. Contents: Prostaglandin E1, Prostaglandin E2, Prostaglandin F1?, 6-keto...
This mixture contains the primary metabolites of prostaglandins (PGs) D2, E2, and F2?. Contents: 13,14-dihydro-15-keto PGD2, 13,14-dihydro-15-keto PGE2, 11?-PGF2?, 13,14-dihydro-15-keto...
Prostaglandin I synthase (PGIS) catalyzes the isomerization of PGH2 to PGI2. PGI2 (prostacyclin) is a potent vasodilator and inhibitor of...
TAF10 is one of many protein factors or coactivators associated with RNA polymerase II activity.{16903} One vial of this peptide...
To be used in conjunction with Cayman’s NAPE-PLD polyclonal antibody (aa 6-20) (Item No. 10306) to block protein-antibody complex formation...
Platelet-activating factor (PAF) is an important lipid mediator involved in inflammation. PAF-acetylhydrolase (PAF-AH) is an extracellular phospholipase A2 which hydrolyzes...
Protein phosphorylation is an important post-translational modification that serves many key functions to regulate a protein’s activity, localization, and protein-protein...
The cyclopentenone prostaglandin HPLC mixture contains all of the major UV-absorbing cyclopentenone prostaglandins and their precursors supplied in methyl acetate....
This mixture contains the characteristic metabolites of both PGI2 and TXA2. Contents: Thromboxane B2, 11-dehydro Thromboxane B2, 6-keto Prostaglandin F1?,...