Territorial Availability: Available worldwide directly through Bertin or your local distributor
Technical Warning: Check the Additional Items Required section of this kit booklet to verify if UltraPure Water (Milli-Q or equivalent) is needed for this assay
- Synonyms
- Correlated keywords
- Product Overview:
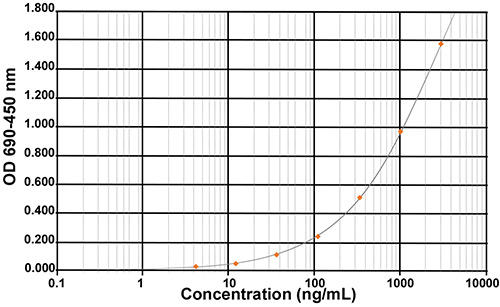
Enzyme ImmunoAssay (EIA) is a technique to detect and quantify antigens (proteins, hormones…) or antibodies in samples. It relies on the ability of an antibody to bind a specific antigen. Either the antibody or the antigen is labelled with an enzyme whose substrate is a chromogen or a fluorogen converted in a measurable product (color or fluorescence).|Enzyme-linked Immunosorbent Assay (ELISA) is a type of EIA using a solid phase (ex: microtiter plate) coated with an antigen immobilizing the molecule to detect. Over the time, scientists have extended the term ELISA to EIAs using an antibody coating the solid phase. That explains why our EIA kits using coated antibodies are also called ELISA kits.|Impurity assessment is a key step during the drug development production of recombinant proteins, including therapeutic proteins. Specific impurities coming from the cells mediating the protein expression, known as Host Cell Proteins (HCP) are generated and need to be removed. This kit is intended for use in assessing relative quantities of E. coli HCP in manufactured or research bioproducts.|Polyclonal antibodies used in this kit have been generated against several strains of E.coli and specifically selected for their recognition of a large spectrum of E. coli proteins. Thus, this kit can be considered as generic and allows a relative-quantitative determination of E. coli HCP in many types of samples, such as samples issued from the purification process (HCP clearance), process control, quality control, or product release.|Using this kit, HCP concentration is measured in ng/ml (HCP equivalent is extrapolated from a standard curve). Conventionally, the HCP content in a product will finally be expressed in ng/mg, where ng represents HCP mass and mg represents the product mass. Note that, contrary to the concentration measurement of the product, the HCP signal is only reflective of antibody binding and does not strictly reflect the mass of HCP.|This kit has been successfully validated for recovery and precision using reconstituted HCP samples and tested against different final products. Given the diversity of final products, all potential matrix effects cannot be known and it is recommended that you test the suitability of the kit for use with your own HCP samples in your laboratory.|This kit should be used as one part of your complete HCP analysis.